Understanding prostate enlargement
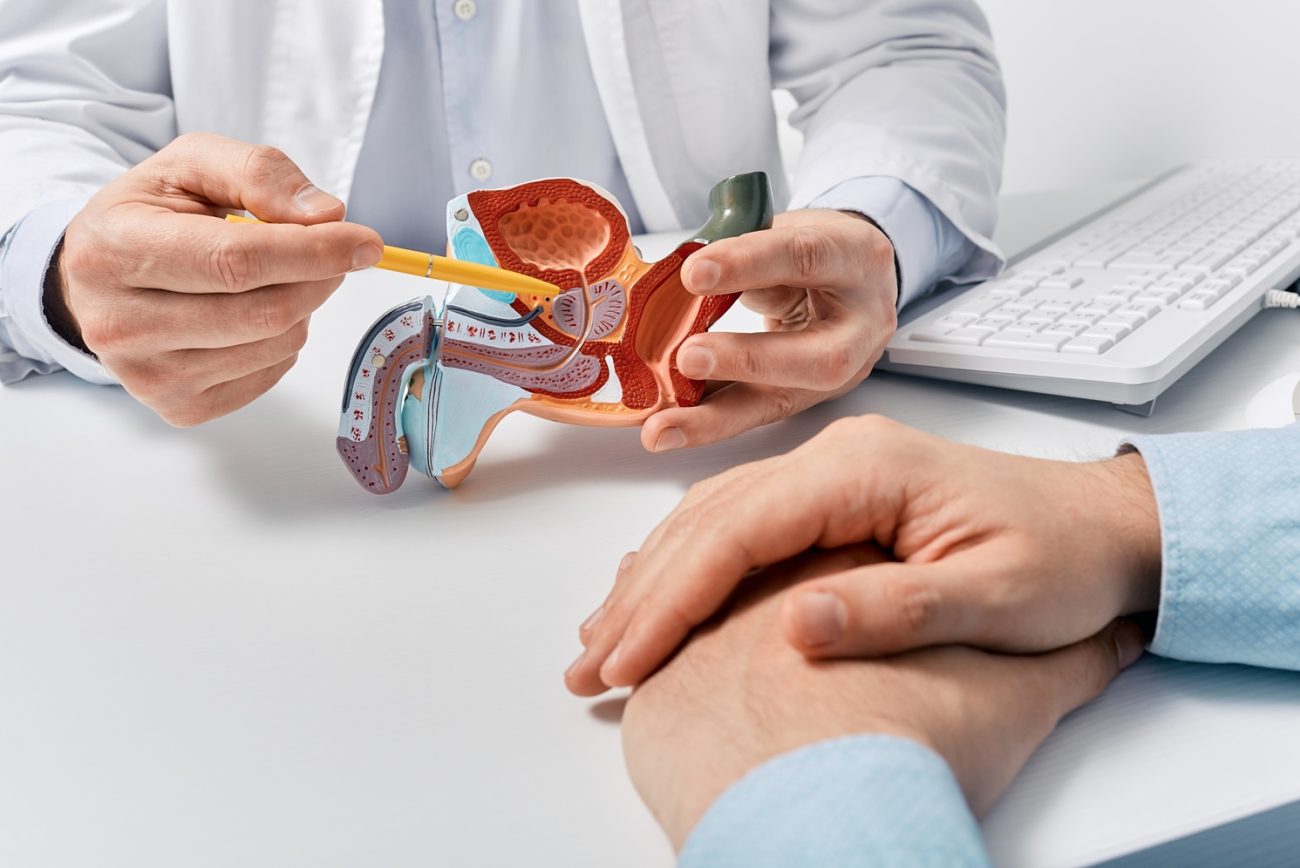
Benign prostatic hyperplasia (BPH) is a non-malignant enlargement of the prostate gland and one of the most common urological conditions affecting people with a prostate (1). The enlargement of the prostate is not always symptomatic, but when it is, it can have a significant impact on quality of life (2). The incidence of BPH increased with age (1).
Worldwide prevalence increases substantially after the age of 50, and in the United Kingdom the pattern is similar. Estimates suggest that over 40% of people with a prostate aged 50–59 show evidence of BPH, rising to around 70–80% aged 70–80 (3).
Clinical symptoms, however, do not always correlate with histological changes. Approximately one-third of men over 50 experience moderate-to-severe lower urinary tract symptoms (LUTS) associated with BPH (1).
Within the UK, BPH represents a major component of primary care. LUTS due to prostate enlargement account for thousands of GP appointments annually, and the condition is associated with significant healthcare expenditure linked to medications (such as α-blockers and 5-α-reductase inhibitors), specialist referrals, and surgical interventions. The ageing population suggests that prevalence will continue to rise (2).
How does benign prostatic hyperplasia (BPH) work?
BPH involves hyperplasia of both stromal and epithelial cells within the transition zone of the prostate. This results in compression of the urethra and changes in bladder dynamics (1,4).

Key elements of pathophysiology include:
- Androgen conversion to dihidrotestosterone (DHT) (1,4): Free testosterone is converted to DHT via the enzyme 5-α-reductase, highly active in prostatic tissue. DHT binds androgen receptors with greater affinity than testosterone, promoting cellular proliferation and inhibiting apoptosis.
- Oestrogen’s pro-inflammatory role: Elevated levels of oestrogen can be pro-inflammatory. In BPH, oestrogen increases stromal growth, fibrosis, and the secretion of growth factors. Increased enzymatic aromatase activity, stimulated by systemic inflammation, drives excess conversion of testosterone to oestradiol. This increases the oestrogen/testosterone ratio and perpetuates prostate enlargement (5,6).
- Chronic inflammation and cytokine activity: Inflammatory cytokines, including TNF-α, IL-6, and IL-8, stimulate cell proliferation, angiogenesis, and extracellular matrix deposition. TNF-α also increases the activity of aromatase enzyme, supporting oestrogenic activity and creating a self-reinforcing cycle of inflammation and hyperplasia (7,8).
- Bladder changes: Long-standing obstruction can lead to detrusor hypertrophy, bladder hypersensitivity, and functional changes such as reduced compliance or impaired contractility. All this contributes to urgency, frequency, and incontinence (1).
This combination of hormonal, inflammatory, mechanical, and neuromuscular factors underpins the clinical manifestations of BPH.
These are risk factors contributing to BPH (9):
- Age over 50, with exponential risk increase with each decade
- Family history of BPH
- Metabolic syndrome, including obesity, hypertension, dyslipidaemia and insulin resistance
- Sedentary lifestyle and poor diet
- Systemic inflammation
Understanding the root causes
The exact cause of BPH remains multifactorial and incompletely understood. However, several interrelated mechanisms have been identified:

Hormonal imbalance
Ageing alters androgen and oestrogen levels. Even though circulating testosterone declines with age, the prostate experiences increased conversion of free testosterone into DHT through 5-α-reductase. DHT is a potent androgen that stimulates prostate cell proliferation (5).
Simultaneously, ageing people with a prostate often exhibit an increased oestrogen-to-testosterone ratio, further exacerbated by aromatase activity. Aromatase converts testosterone into oestradiol-17β, an oestrogen that can upregulate growth factors and inflammatory signalling in the prostate (4).
Chronic inflammation
Persistent low-grade inflammation is increasingly considered both a cause and a result of prostatic enlargement. Inflammatory cytokines, including TNF-α, promote aromatase activity, perpetuating a cycle of hormonal imbalance and cellular proliferation. This chronic inflammatory environment contributes to fibrosis, smooth muscle hyperplasia, and stromal expansion (1,7).
Metabolic factors
Metabolic syndrome, insulin resistance, and obesity are associated with increased risk of BPH and LUTS (10). Hyperinsulinaemia can stimulate sympathetic nervous system activity and increase prostatic smooth muscle tone, while adipose tissue increases aromatase activity, promoting hormonal imbalance.
Genetics
Evidence suggests that familial clustering of BPH may be partly due to variations in androgen metabolism and growth factor expression. Epigenetic mechanisms, including DNA methylation and altered gene expression in prostatic stromal and epithelial cells could also be playing a role (11).
Signs and symptoms

Symptoms of BPH happen as a result of mechanical obstruction from prostate enlargement and dynamic changes in smooth muscle tone around the bladder neck and urethra. Patients may present with classic LUTS, which are divided into voiding (obstructive) and storage (irritative) symptoms.
Common symptoms include (1,12):
- Increased urinary frequency, particularly during the night (nocturia)
- Urgency, sometimes accompanied by urge incontinence
- Hesitancy, a delay before initiating voiding
- Weak or intermittent urinary flow
- Sensation of incomplete bladder emptying
- Post-micturition dribbling
Assessment tools used in clinical practice include the International Prostate Symptom Score (IPSS), which grades severity, and digital rectal examination (DRE), typically revealing a smooth, symmetrically enlarged prostate (13). In some cases, urodynamic studies, urinary flow tests and prostate-specific antigen (PSA) levels may be assessed to rule out malignancy or alternative pathology (14).
Herbs for prostate enlargement

Key strategies to improve symptoms of BPH include addressing hormonal balance, inflammation, and urinary function through the use of aromatase-modulating plants, anti-inflammatory herbs and bladder tonic herbs (22).
Aromatase-modulating plants
Phytoestrogens are chemical compounds in plants that act as aromatase inhibitors by decreasing aromatase gene expression, inhibiting the aromatase enzyme itself, or in some cases acting at both levels of regulation (15). Aromatase enzyme converts testosterone into oestrogen.
Testosterone has an anti-inflammatory effect, and oestrogen, although at times anti-inflammatory, generally has a pro-inflammatory effect. Pro-inflammatory cytokines (e.g., TNF-alpha) circulating in the body stimulate aromatase activity, therefore promoting the conversion of testosterone into a pro-inflammatory type of oestrogen. This is why persistent inflammation in the body is going to contribute to the aromatisation of testosterone into oestrogen.
Phytoestrogen containing plants (16):
- Red clover (Trifolium pratense) contains isoflavone biochanin A, an isoflavone that reduces aromatase gene expression (17).
- Hops (Humulus lupulus) contains prenylated flavonoids, which modulate aromatase activity and can influence androgen metabolism (18).
- Soy (Glycine max) contains the isoflavone genistein, a phytoestrogen that exhibits both aromatase inhibition and anti-inflammatory effects (16).
- Lemon (Citrus x limon), bitter orange (Citrus aurantium) and other citrus species have peel rich in naringenin, naringin and quercetin, all of which are aromatase inhibitors (19).
Foods with aromatase-modulating activity include bay leaf, mint (Mentha spp.), garlic (Allium sativum), saffron (Crocus sativus), and Brassica family vegetables, particularly broccoli sprouts (16).
Anti-inflammatory herbs
Given the central role of TNF-α and inflammatory cytokines in BPH pathophysiology, herbs that reduce inflammation are a valuable therapeutic addition.
- Saw palmetto (Serenoa repens) reduces 5-α-reductase activity, improves urinary flow, and has anti-inflammatory actions (20).
- Nettle (Urtica dioica radix) root can modulate sex hormone-binding globulin (SHBG) and reduce prostatic inflammation (21).
- African prune tree (Pygeum africanum) improves LUTS through anti-inflammatory and anti-proliferative actions (21).
- Turmeric (Curcuma longa) inhibits pro-inflammatory cytokines like NF-κB and TNF-α.
Urinary tonics
These herbs support urinary function by strengthening the bladder and can help reduce irritative symptoms (22). Examples include corn silk (Zea mays), which is demulcent and soothing to the urinary tract, small-flowered willow herb (Epilobium parviflorum), traditionally used for prostatitis and BPH, and varuna (Crataeva nurvala), a well-known Ayurvedic herb traditionally used to support urinary tract health which helps strengthen the tone of the bladder wall and detrusor muscle (22).
Key herbs for treating prostate enlargement

Saw palmetto (Serenoa repens)
Saw palmetto has been traditionally used to manage symptoms of BPH and has been widely studied for this purpose. Its primary active constituents are found in a liposterolic extract, which includes free fatty acids (FFAs) and phytosterols (22). Clinical trials have most commonly used a dosage of 320 mg per day (23,24). Evidence from several studies suggests that saw palmetto exhibits mild 5-alpha-reductase inhibitory activity (23).
In addition, saw palmetto demonstrates anti-inflammatory effects by reducing pro-inflammatory cytokines such as IL-1 and TNF-α. A study reported a statistically significant reduction in BPH symptoms associated with saw palmetto intake (p < 0.006) (24).
However, the evidence regarding its effectiveness remains conflicting. A meta-analysis concluded that saw palmetto was more effective than placebo in reducing nocturia (p < 0.05) (23). In contrast, a Cochrane Review found no significant improvement in BPH symptoms (25). It is important to note that many of the trials included in the Cochrane Review evaluated saw palmetto as a monotherapy and used preparations of varying quality. Standardisation to free fatty acid content appears to be a key factor influencing outcomes.
The available evidence suggests that saw palmetto may be most effective when used synergistically with other medicinal plants, such as nettle root and African prune tree (26).
Nettle root (Urtica dioica radix)
Nettle root is another medicinal plant traditionally used for treatment of BPH. It contains bioactive compounds such as polyphenols and quercetin, which have a significant anti-inflammatory action (23). Clinical trials have demonstrated good efficacy when nettle root is used in combination with a standardised free fatty acid (FFA) extract of saw palmetto (26). In these studies, participants experienced reduced urinary frequency, improved urinary flow, and decreased levels of sex hormone–binding globulin (SHBG).
Nettle root also appears to inhibit aromatisation, the conversion of free testosterone into pro-inflammatory oestrogen, which may further contribute to its benefits in BPH (27). This action could be attributed partly to lignans in nettle root, which bind to SHBG, decreasing DHT conversion (28). Additionally, clinical data indicate that nettle root is more effective when combined with an extract of Pygeum africanum than when used as a monotherapy (29).
African prune tree (Pygeum africanum)
African prune tree been studied for the treatment of BPH (30). Although its exact mechanism of action is not fully understood, evidence suggests that it has mild 5-alpha-reductase inhibitory activity, anti-inflammatory effects, and the ability to inhibit growth factors involved in prostatic enlargement (30). Clinical studies have shown that Pygeum africanum is particularly effective when used in combination with nettle root, further supporting the importance of synergistic herbal therapies in BPH management (21).
Holistic solutions

A Mediterranean diet has been associated with improved outcomes BPH. A diet rich in vegetables, fruits, nuts, legumes, whole grains, fish, and olive oil while low in red meat, provides high levels of antioxidants, carotenoids, vitamin C, and polyphenols (31). These nutrients are linked to a lower incidence of BPH and reduced symptom severity.
Reducing caffeine and alcohol intake can also improve urinary urgency and frequency, while increased consumption of cruciferous vegetables like broccoli Brussels sprouts supports oestrogen metabolism and detoxification (31,32).
Improving insulin sensitivity is also important to symptom management (33). Regular physical activity, particularly moderate aerobic exercise, reduces systemic inflammation, sympathetic nervous system activity, and lower urinary tract symptoms.
Chronic stress, which increases sympathetic tone and bladder tension, can worsen voiding symptoms, so relaxation techniques such as breathing exercises, yoga, tai chi, and meditation can support parasympathetic activity and improve urinary function (34).
References
- Chughtai B, Forde JC, Thomas DDM, et al. Benign prostatic hyperplasia. Nat Rev Dis Primers. 2016;2:16031. https://doi.org/10.1038/nrdp.2016.31
- Speakman M, Kirby R, Doyle S, Ioannou C. Burden of male lower urinary tract symptoms suggestive of benign prostatic hyperplasia: focus on the UK. BJU Int. 2015;115(4):508–519. https://doi.org/10.1111/bju.12745
- Chen X, Yang S, He Z, et al. Comprehensive analysis of the global, regional, and national burden of benign prostatic hyperplasia from 1990 to 2021. Sci Rep. 2025;15(1):5644. https://doi.org/10.1038/s41598-025-90229-3
- Roehrborn CG. Pathology of benign prostatic hyperplasia. Int J Impot Res. 2008;20(suppl 3):S11–S18. https://doi.org/10.1038/ijir.2008.55
- Ho CK, Nanda J, Chapman KE, Habib FK. Oestrogen and benign prostatic hyperplasia: effects on stromal cell proliferation and local formation from androgen. J Endocrinol. 2008;197(3):483–491. https://doi.org/10.1677/joe-07-0470
- Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011;8(1):29–41. https://doi.org/10.1038/nrurol.2010.207
- Fibbi B, Penna G, Morelli A, Adorini L, Maggi M. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int J Androl. 2010;33(3):475–488. https://doi.org/10.1111/j.1365-2605.2009.00972.x
- Bouraoui Y, Ricote M, García-Tuñón I, et al. Pro-inflammatory cytokines and prostate-specific antigen in hyperplasia and human prostate cancer. Cancer Detect Prev. 2008;32(1):23–32. https://doi.org/10.1016/j.cdp.2008.02.007
- Parsons JK. Benign prostatic hyperplasia and male lower urinary tract symptoms: epidemiology and risk factors. Curr Bladder Dysfunct Rep. 2010;5(4):212–218. https://doi.org/10.1007/s11884-010-0067-2
- Li J, Peng L, Cao D, Gou H, Li Y, Wei Q. The association between metabolic syndrome and benign prostatic hyperplasia: a systematic review and meta-analysis. Aging Male. 2020;23(5):1388—1399. https://doi.org/10.1080/13685538.2020.1771552
- Daryabari SS, Fendereski K, Grimes MD, et al. A scoping review of the role of heritability and environmental exposures in the development and severity of benign prostatic hyperplasia. Transl Androl Urol. 2025;14(8):2439–2455. https://doi.org/10.21037/tau-2025-342
- NHS. Benign prostate enlargement. https://www.nhs.uk/conditions/prostate-enlargement/. Published 2020. Accessed October 10, 2025.
- American Urological Association Symptom Index (AUA-SI)/International Prostate Symptom Score (IPSS). https://www.mdcalc.com/calc/10462/american-urological-association-symptom-index-aua-si. Accessed October 10, 2025.
- Porru D, Jallous H, Cavalli V, Sallusto F, Rovereto B. Prognostic value of a combination of IPSS, flow rate and residual urine volume compared to pressure-flow studies in the preoperative evaluation of symptomatic BPH. Eur Urol. 2002;41(3):246-249. https://doi.org/10.1016/s0302-2838(02)00021-0
- Lephart ED. Modulation of aromatase by phytoestrogens. Enzyme Res. 2015;2015:594656. https://doi.org/10.1155/2015/594656
- Balunas MJ, Kinghorn AD. Natural compounds with aromatase inhibitory activity: an update. Planta Med. 2010;76(11):1087–1093. https://doi.org/10.1055/s-0030-1250169
- Wang Y, Man Gho W, Chan FL, Chen S, Leung LK. The red clover (Trifolium pratense) isoflavone biochanin A inhibits aromatase activity and expression. Br J Nutr. 2008;99(2):303–310. https://doi.org/10.1017/s0007114507811974
- Monteiro R, Becker H, Azevedo I, Calhau C. Effect of hop (Humulus lupulus L.) flavonoids on aromatase (estrogen synthase) activity. J Agric Food Chem. 2006;54(8):2938–2943. https://doi.org/10.1021/jf053162t
- El-Kersh DM, Ezzat SM, Salama MM, et al. Anti-estrogenic and anti-aromatase activities of citrus peels major compounds in breast cancer. Sci Rep. 2021;11(1):7121. https://doi.org/10.1038/s41598-021-86599-z
- Habib FK. Serenoa repens: the scientific basis for the treatment of benign prostatic hyperplasia. Eur Urol Suppl. 2009;8(13):887–893.
- Hartmann RW, Mark M, Soldati F. Inhibition of 5α-reductase and aromatase by PHL-00801 (Prostatonin®), a combination of Pygeum africanum and Urtica dioica extracts. Phytomedicine. 1996;3(2):121–128. https://doi.org/10.1016/s0944-7113(96)80025-0
- Bone K, Mills S. Principles and practice of phytotherapy: modern herbal medicine. Elsevier Health Sciences; 2013.
- Boyle P, Robertson C, Lowe F, Roehrborn C. Updated meta-analysis of clinical trials of Serenoa repens extract in the treatment of symptomatic benign prostatic hyperplasia. BJU Int. 2004;93(6):751-756. https://doi.org/10.1111/j.1464-410x.2003.04735.x
- Navarrete RV, Cardoso JG, Barat A, Manzarbeitia F, Farré AL. BPH and inflammation: pharmacological effects of Permixon on histological and molecular inflammatory markers. Eur Urol. 2003;44(5):549–555. https://doi.org/10.1016/s0302-2838(03)00368-3
- Tacklind J, MacDonald R, Rutks I, Stanke JU, Wilt TJ. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2009;(2):CD001423. https://doi.org/10.1002/14651858.cd001423.pub2
- Bondarenko B, Walther C, Funk P, Schlafke S, Engelmann U. Long-term efficacy and safety of PRO 160/120 (a combination of Sabal and Urtica extract) in patients with lower urinary tract symptoms. Phytomedicine. 2003;10(suppl 4):S53. https://doi.org/10.1078/1433-187x-00352
- Ganßer D, Spiteller G. Aromatase inhibitors from Urtica dioica roots. Planta Med. 1995;61(2):138–140. https://doi.org/10.1055/s-2006-958033
- Schöttner M, Ganßer D, Spiteller G. Lignans from the roots of Urtica dioica and their metabolites bind to human sex hormone binding globulin. Planta Med. 1997;63(6):529–532. https://doi.org/10.1055/s-2006-957756
- Melo EA, Bertero EB, Rios LA, Mattos D Jr. Evaluating the efficiency of a combination of Pygeum africanum and stinging nettle extracts in treating benign prostatic hyperplasia: a double-blind, randomized, placebo-controlled trial. Int Braz J Urol. 2002;28(5):418–425.
- Ishani A, MacDonald R, Nelson D, Rutks I, Wilt TJ. Pygeum africanum for the treatment of patients with benign prostatic hyperplasia: a systematic review and quantitative meta-analysis. Am J Med. 2000;109(8):654–664. https://doi.org/10.1016/s0002-9343(00)00604-5
- Rohrmann S, Giovannucci E, Willett WC, Platz EA. Fruit and vegetable consumption, intake of micronutrients, and benign prostatic hyperplasia in US men. Am J Clin Nutr. 2007;85(2):523–529. https://doi.org/10.1093/ajcn/85.2.523
- Russo GI, Broggi G, Cocci A, et al. Relationship between dietary patterns with benign prostatic hyperplasia and erectile dysfunction: a collaborative review. Nutrients. 2021;13(11):4148. https://doi.org/10.3390/nu13114148
- Vikram A, Jena G, Ramarao P. Insulin-resistance and benign prostatic hyperplasia: the connection. Eur J Pharmacol. 2010;641(2-3):75–81. https://doi.org/10.1016/j.ejphar.2010.05.042
- McVary KT, Rademaker A, Lloyd GL, Gann P. Autonomic nervous system overactivity in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2005;174(4 pt 1):1327–1333. https://doi.org/10.1097/01.ju.0000173072.73702.64




























